
Neural Temporal Codes for Representation of Information in the Central Nervous System
Accepted 27 June 2008
KEYWORDS
neural codes, temporal codes, information
Abstract
The goal of neural coding research is to understand how the brain uses adaptive neural signals to represent and transmit information. This review surveys recent evidence concerning the nature of representation implemented by neural circuits. We contrast rate coding with different forms of temporal codes, arguing that at the level of a single neuron, this dichotomy is a simple problem of demonstrating the optimal window size for integration that could carry the behaviorally relevant information. Also, we draw on examples from vision and from other systems to illustrate how information may be coded hierarchically along a pathway. More-over, we stress the importance of higher-order interactions, such as the relative timing of first-spike latencies from ensembles of neurons, which gives the cortex a potentially large immense representational capacity. Evidence derived from coupling massive multirecording techniques and 3D real-time voltage and/or magnetic imaging should yield enough information to reveal a more realistic picture of neural codes and network interactions. (Cogn Critique 1: 1-30, 2008)
INTRODUCTION
To respond adaptively, the organism constructs dynamic neuronal representations embodied in some sort of inner format that facilitates the selection of behavior. A representation is a message that uses neural states or processes, defined by two principal and overlapping characteristics: content and function. Content is the message that a representation carries, for example, what the signal signifies about a sensory input; thereby the modality within which sensation is experienced carries information about the nature of the stimulus as a labeled line. Thus, information could be defined as a message transmitted and usually transformed between receivers. Function is the effect that the signal can have on cognitive processes and the resultant behavior. Therefore, the signal must have a statistical relation to both the input and the output; consequently not all signals involve causal representations temporally coupled with the ongoing process measured (deCharms and Zador 2000; Eagleman and Churchland, in press). Although there are various candidate vehicles of representation, discrete pulse events, known as action potentials or spikes in individual neurons, are an initial plausible candidate, given that spikes can be configured in a vast repertoire of patterns (Fig. 1).
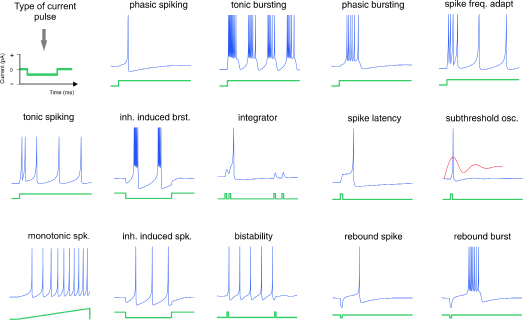
Figure 1. Diversity and complexity of neuronal electrical behavior (blue) in response to different injected current steps (green). Here is shown a type of spike-alphabet emitted by neurons that afford various ways of firing patterns that are constrained by the neuronal biophysical properties. (Reproduced with permission from www.izhikevich.com.)
Neurons use significant amounts of energy (3.9 x 1020 ATP. min-1) to support ionic movements associated with these spikes (Lennie 2003). Neurons have the remarkable property to propagate these action potentials, which can travel down nerve fibers in an all-or-none fashion. For a given neuron, the amplitude and duration of a single spike are quite constant, and its response resembles the binary code used in computer science. Therefore, information is carried through the temporal succession of action potentials from a neuron, not through their magnitude or duration. However, how can we be certain that these action potentials can represent behavioral information? There are two main approaches to test the representational role of a signal: co-variation of the signal recorded with a behavioral event, and, mimicking (by microstimulation) of the candidate signal that should lead to a measurable perceptual or motor effect. In any case, spike activity fulfilled both these criteria in a number of examples described elsewhere (Parker and Newsome 1998; Romo et al. 1998; Di Lorenzo et al. 2003; Cohen and Newsome 2004). Another feature of spiking neurons is the variability of their responses elicited by the same input over many trials. For example, fluctuations in the mean spike count over a fixed time and irregularities of inter-spike intervals of a single neuron in response to identical stimulations may be introduced by non-linear integration during spike generation or synaptic transmission at all levels of a processing pathway. However, the variability in the responses is not only signaling noise, it can be also a source of information. Nevertheless, although individual neurons reliably fire action potentials, information is sorted and processed by neural networks capable of rapidly handling large amounts of information. The nervous system probably has developed structural and functional features that exploit the temporal variation of action potentials to represent information, mediating perceptual synthesis and adaptive sensorimotor integration (Friston 1997). An intuitive question at this point is, how are these spikes used by the brain to code information?
Representational signals can be carried by neurons using a number of potential codes. Thus a “neural code” could be understood as a system of symbols and rules by which information is carried (Paillard 1983; Halpern 2000). For single neurons, these codes include the firing rate of the cell, which is the total number of spikes counted in an arbitrary time window, and the temporal structure of neuronal spike trains, where the exact time of every spike is informative (Fig. 2).
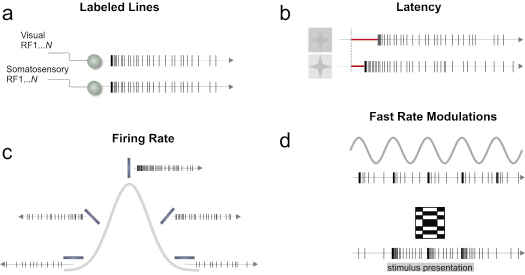
Figure 2. Predominant single neuron coding schemes. (a) By specific receptive field and modality through connectivity a given neuron sends a signal related to a particular message. (b) Differences in latency signaling the contrast changes of visual stimuli. (c ) A tuning curve is shown where a neuron responds preferentially to a particular stimulus attribute. (d) Local fast modulations in a spike train to code time-varying signals (top panel) or a static spatial stimulus (bottom panel). These fundamental modes of coding are not mutually exclusive and can be combined to form more complex coding schemes at the population level.
For larger populations of neurons (Fig. 3), coordinated codes involve the relationships among the activities of a number individual neurons, whereas independent codes involve the pooling of distributed signals from cell populations (Georgopoulos et al. 1986). Through measurements of the contents and functions of neuronal signals, these signals can be directly linked to the behavioral and cognitive processes they mediate (Parker and Newsome 1998; deCharms and Zador 2000; Erickson 2001). Our aim in this paper is to review some examples, where numerous temporal coding schemes are evidenced, tracing a spatial evolution of the transformation of the coding scheme at different levels in the brain, particularly at the neocortex stage in the visual system. This synthesis may help bring out consistencies, which could shed some light on the functional significance of the current coding theories.
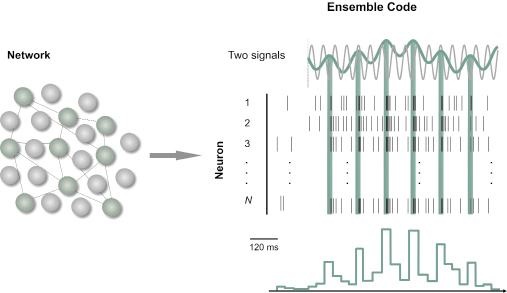
Figure 3. An ensemble temporal code. Spike trains from functionally connected neurons were pooled to create a peri-stimulus time histogram (PSTH) in response to the peaks of one of two oscillating signals.
The Rate vs Timing conundrum
Information in the brain is encoded by patterns of trains of action potentials generated by neural populations. These action potential patterns show specific topographic distributions across a neural circuit and temporal relations among active channels. In recent years, the debate in the literature revolves around the significance of temporal coding vs. rate coding. However, this seems rather artificial since the brain has been shown to employ both rate coding and temporal coding to varying degrees in different parts of the nervous system, depending on task demands and features of the stimulus perceived (Stein et al. 2005). Then we should keep in mind that these are not mutually exclusive coding proposals (Hubel and Wiesel 1959; Mountcastle 1980; Reich et al. 2000; Salinas et al. 2000; Moore et al. 2001; Hess et al. 2003). Neuroscientists often describe the behavior of spiking neurons in terms of firing rate of individual cells, where spike events are integrated over a fixed time window, and where only the mean frequency of spikes matters (Aertsen and Braitenberg 1996). Correspondingly, the mean firing rate is not a well-defined property of a sequence of events, assuming that spiking neurons generate sequences of pulses that differ from each other only with respect to their relative occurrence times. From this reasoning emerges the proposal of an extra channel of information embedded in the precise temporal structure of spike trains, exploited by the brain as a temporal code. Temporal coding, in its broadest sense, refers to two types of problems. First, it assigns importance to the precise timing and coordination of spikes for feature information coding, expanding the brain possibilities of stimulus representation. Second, the brain could represent time itself as a variable, solving sensorimotor problems such as interval duration and motion discrimination, as well as complex forms of sensory processing, from speech recognition to bimanual coordination to playing the piano (Mauk and Buonomano 2004). This distinction between spike timing and time representation will be crucial in the following sections, where we will deal only with the first form of temporal coding.
Spike time integration or the queuing for the
“meaning”
Let us contrast the observation of periodicity with the notion of rate coding. In this way, instantaneous firing rate is the probability that a spike will occur in a small time window; if we make the window larger the probability will be larger, and we will not be able to discern the periodicity of the firing given the poor resolution of the kernel. However, by using short enough time windows, a temporal structure of the response can be revealed, and it is possible to investigate the spatio-temporal clues in the signal itself (Dayan and Abbott 2001). On the other hand, with large time windows the temporal structures become secondary, and the number of spikes in the window is what matters for carrying information. Consequently, the problem of rate versus temporal coding in this scheme is a simple problem of demonstrating which window of integration can carry the behaviorally relevant information in a robust fashion.
In a recent study in which psychophysical and neurophysiological experiments were conducted in monkeys trained in a vibrotactile discrimination task, researchers propose a minimal time window during which the firing rate was successfully integrated in the primary somatosensory cortex. They report a 250-ms weighted window that covaries with the monkey psychophysical performance (Luna et al. 2005). From the decoding perspective, the read-out rule using a simple firing rate from independent neurons, even with an optimized kernel, yields questionable and probably overestimated results, because the brain uses multiple neurons instead of many trials. The reasoning is as follows: if we take into account the latencies of information processing in the same system under the same vibrotactile stimuli, we have neurons in the primary somatosensory cortex (SI) that respond with a latency of 20.2 ± 4.5 ms (mean ± SEM), those in the secondary somatosensory cortex (SII) with a latency of 29.9 ± 7.4 ms, and those in the medial premotor cortex (MPC) with a latency of 67 ± 13 ms (Hernandez et al. 2002). Given the limited trial-based firing rates discussed above, a weak point with this measure becomes apparent when considering (a) that the time windows involved are typically quite long, 250-500 ms, and (b) that the time needed for several spikes to accumulate in order to estimate the firing rate is usually longer than the time needed for most perceptual or behavioral processes (Guyonneau et al. 2004). These considerations are in conflict with the idea of a firing rate code measured across trials using independent pooled neurons. For example, it has been shown that neurons in the infero-temporal cortex can be highly selective for stimuli such as faces, can respond only 80-100 ms after stimulus onset (Rolls 2000), and that the primate visual system can analyze complex natural scenes in only 100-150 ms (Thorpe et al. 1996). The studies above suggest that information arrives with different jitters, even in the same area, by recurrent and parallel connections (Schmolesky et al. 1998), and that neurons seem to weight the first spikes of a train and use shorter integration times than the typical time windows used to estimate firing rates. In this respect, the cortex likely deals with this problem by estimating the rate or timing of coordinated spikes using several active neurons and their jitter times, instead of several independent trials.
Variability of neuronal responses
Deciphering the neural code requires an understanding of the biophysical constraints, which limit the temporal precision or reliability of neuronal spike trains (Softky and Koch 1993; Steinmetz et al. 2001). The neuronal response variability has been characterized by a count and interval statistics. Two measures of spiking responses are commonly employed: the interspike-interval distribution and the spike-count distribution (Dayan and Abbott 2001). In numerous studies, especially in the visual systems of vertebrates, the spike-count variability has been quantified by the Fano factor (FF = variance/mean ratio of the spike counts), and as a standard quantification of the interspike-interval (ISI) variability, the coefficient of variation (CV = standard deviation/mean ratio of the interspike intervals) is calculated (Tolhurst et al. 1983; Softky and Koch 1993; Vogel et al. 2005). While the FF reflects the response reliability for multiple stimulus presentations, the variability of an ongoing neuronal response is expressed by the CV. Thus, the relation between these two measures may help characterize a neuron’s spiking response (Werner and Mountcastle 1963; Stevens and Zador 1998). The neuronal spike train variability often resembles the variability expected from a Poisson process, in which each event occurs independently of the occurrence of other events. For a Poisson process, the variance of the number of events counted in a set of equal time intervals is equal to the mean count across the intervals. For cortical neuronal responses, when an identical visual stimulus is presented for several repetitions, the variance of the neural spike count has been found to exceed the mean spike count by a ratio of 1–1.5 wherever in the cortex has been measured, approaching the Poisson model (Softky and Koch 1993; Shadlen and Newsome 1994; Lee et al. 1998; Shadlen and Newsome 1998). Equally, in a hypothetical Poisson event train, the time intervals between successive events are independent and exponentially distributed. Note that the irregularity of the distribution of counts in a particular time interval derives directly from the irregularity of spike timing. The interspike interval distribution for many cortical neurons can be fitted by an exponential probability density function, but the CV values are only valid if the response rate is a constant (Shadlen and Newsome 1998; Christodoulou and Bugmann 2001). In certain cases, the FF and the CV are related by the equation: FF = CV2. The main requirement is that every ISI in a spike train be statistically independent of every other ISI, showing that the spike train follows a Poisson behavior (Stevens and Zador 1998). But under these assumptions, it is possible that spike reliability, precise latency, and high speed rate modulation in the overall neuronal behavior were not properly evaluated when using a Poisson model. One line of reasoning is that a significant source of variability under identical conditions is encoding hidden contextual variables not measured by the experimenter. This internal ongoing activity has been shown to contribute at least in part to the variability commonly reported in cortical responses, as Arieli et al. suggested using optical recordings on the primary visual cortex of anesthetized cats. Inferring lack of precision at the cortical level of processing from these rough measures could be tricky, given the capacity of the cortex to manage multidimensional variables.
Other experimental approaches revealed that in vitro experiments the FF values were consistently lower than those observed in vivo, reinforcing the previous observations that isolated inputs cannot account for the high variability observed in vivo (Stevens and Zador 1998; Knoblauch and Palm 2005). In an in vitro study the temporal pattern of the response of pyramidal neurons to injected current was found to be unreliable when the injected current was constant, but highly reliable when the input current fluctuated and contained high-frequency components. This study demonstrated explicitly the difference between the irregularity of the spike pattern as opposed to the reliability or accuracy of spike timing, and it also highlighted the fact that natural stimuli are noisy and contain sharp transitions (Mainen and Sejnowski 1995). According to this view, the response variability of cortical neurons seems to be a property of synaptic connections, both inhibitory and excitatory, rather than the neurons themselves (Tolhurst et al. 1983; Holt et al. 1996; Movshon 2000). And given that the response variability increases from low values in primary neural processing stages, to greater values in higher processing structures, one of the remarkable sources of central and widespread variability seems to come from intracortical connections, pointing again to the role of the nature and topology of synaptic inputs on the capacity of processing variables (Holt et al. 1996; Kara et al. 2000; Movshon 2000). One example of this was the study by Kara et al. (2000) in which they recorded simultaneously from the retina, LGN and cortex of anesthetized cats in response to a drifting sine-grating stimulus. They found a generalized low variability and a progressive increase from retina to cortex, with FF mean values of 0.15 for retinal ganglion cells, FF = 0.32 for LGN cells and FF = 0.55 for cortical neurons.
It is important to note that these two estimates of variability suffer from a possible drawback: when measuring variability over time, it is conceivable that the outcome is a misleading picture of neuronal variability. Comparing the magnitude of variability along successive stages in a sensory pathway might be problematic since there is evidence that the observed variability could be caused by internal states of the brain related to attention, expectation, motivation, or to other percepts not correlated with the ongoing variable (Abeles et al. 1995; Kenet et al. 2003; Ronacher et al. 2004; Knoblauch and Palm 2005). These internal states could change on a relatively fine time scale (tens to hundreds of milliseconds) and could play an important role in cortical function (Shadlen and Newsome 1998; Kenet et al. 2003).
The signal in the quorum
The problem of computation resolution can be overcome at the level of populations, such that the population rate of an ensemble of neurons can be estimated on a time scale even shorter than the interspike intervals of the individual neurons (Knight 1972; Sakurai 1996). The output of an ensemble can be described by an instantaneous population rate, estimated by the number of spikes emitted by the entire ensemble in small time intervals divided by the number of neurons (Rieke 1997; Dayan and Abbott 2001). Using this measure, recent studies in the human somatosensory system have demonstrated that the relative timing of the first spikes in individual units of ensembles of tactile afferents from the fingertip conveys sufficient information to discriminate four directions of fingertip force and three different shapes of the surfaces contacting the tip. The information is available more promptly than would be possible by the fastest rate code and quickly enough to account for the speed observed in natural object manipulations (Johansson and Birznieks 2004). A code based on the relative timing of first spikes in neuronal ensembles has also been discussed and analyzed theoretically in relation to fast object categorization in central vision (Thorpe et al. 2001). However, given that it is difficult to access hierarchical latencies in cortical areas, researchers have used averaged neuronal responses in order to obtain meaningful signals correlated with the variable of interest.
On the other hand, recent studies have revealed that the timing of individual spikes can represent with remarkable accuracy the time structure of rapidly varying stimuli, such as movement within a visual scene (Rieke 1997), or the coding of naturalistic sounds in central areas of birds (Wright et al. 2002). How should we represent a spike train in order to visualize the neuron’s behavior or to analyze its role in neural computation? To answer this question, Panzeri and co-workers varied the resolution at which the spike times were binned, and computed the average mutual information across stimuli (whisker deflection) as a function of bin size in the rat somatosensory cortex. They found that information increases as bin size is decreased, and the shortest bin that could be robustly estimated was 2.5 ms (Panzeri et al. 2003). This finding suggests that the quantification of the information depends on the temporal structure of the spike train evoked. The critical factor was that the relative latency between the response to the principal whisker and that to surrounding whiskers is preserved in the trial-to-trial variability in first spike time. Therefore small bins under this condition can extract a precision profile from firing rates, based on population of neurons that can account for stimulus discriminability. The same group noted that the total information present when the spikes were considered individually exceeded 100% of that present in the full spike train, indicating that spikes subsequent to the first one were partly redundant. This observation emphasizes the significance of the timing of the first spike, which contributes to the coding of a spatial, behaviorally relevant feature of peripheral events detected by whisker deflections. Moreover, for each time step in the 0–40 ms interval, the first spike accounted for essentially all of the information in firing rate modulation (Panzeri et al. 2001). The same was true for neurons recorded in the secondary auditory cortical field of anesthetized cats in response to noise bursts presented from different azimuthal locations. It was found that the proportion of information about azimuthal source location transmitted by first spike latencies averaged 89% of that of full spike patterns (Furukawa and Middlebrooks 2002).
Although this is only a first approximation, the inevitable conclusion is that small numbers of spikes and more importantly their time of occurrence are capable of contributing to robust stimulus representation using assemblies of functionally correlated neurons. Note that this type of population coding has been reported only in the initial nodes of the sensory hierarchy.
TIMING IN SENSORY SYSTEMS: BASIC FLOOR PLAN
The search for representations in the brain begins with visualizing the brain as acquiring information about the organism’s own body and its environment that can, in turn, be used to guide behavior. Probably the first approximation that followed the same line of reasoning dates back to the classic early work of Lord Edgar C. Adrian, who performed electrical recordings in numerous sensory systems and even explored muscle properties. Adrian put forward three main principles that helped to develop the current ideas in neural coding. The first observation established that individual neurons generate stereotyped all-or-none responses that propagate along the cell axon; the phenomenon is highly conserved among species. This means that information travels and can be read only through the arrival of electrical signals. The next important concept relates to adaptation, or desensitization, i.e. the decline in neural responses as function of time when a constant energy is applied. This principle was demonstrated when the mean firing rate of the muscle stretch receptor decreased as a function of elapsed time when a constant weight load was applied to it. The third contribution comes from the recognition that the variation in frequency of the discharges carried information about stimulus intensity (Adrian 1928). These ideas served as a basis for subsequent research on sensory neurons (Galambos and Davis 1948; Hubel and Wiesel 1959; Werner and Mountcastle 1963). Evidence for stimulus-related spike timing patterns exists in nearly every sensory modality. Such information can potentially be utilized for representation of stimulus qualities, localization of sources, and perceptual grouping. In what follows, we briefly review spike-time coding schemes and possible driving signals in various stages of the visual processing pathway.
Visual system
A visual scene is conveyed first at the level of the retina, which performs a significant amount of processing. The visual signal is integrated from a neural population of 108 photoreceptors into just 106 ganglion cells (the output of the retina) forming optic nerve fibers that transmit in parallel to subsequent circuits (Meister and Berry 1999). The retina has the salient property of highly reliable responses that vary with the effective contrast. For example, Berry and co-workers (1997) observed that in both salamander and rabbit the ganglion cells respond to Gaussian flicker intensity at discrete periods of firing, with a jitter as low as 4.4 ms at the highest contrast (35%) rising to 14 ms at the lowest (2.3%). They also noted that many ganglion cells, when driven by a broad mixture of fast and slow stimulus waveforms, respond to a small subset of stimulus features with high precision in the first few spikes and simply do not respond to the others (Berry et al. 1997). A central assumption is that the retinal code can be formulated by describing the responses of individual ganglion cells based on their discharge rate; however, retinal ganglion cells engage in significant patterns of concerted activity that cannot be derived from any single-neuron description. This coordinated activity has been suggested to be an extra channel of information (Castelo-Branco et al. 1998; Levine et al. 2002). Thus, it has been recognized that neighboring ganglion cells in vertebrate retina typically show an increased probability of firing together within some relatively narrow temporal window, much greater than expected by chance (DeVries 1999; Levine et al. 2002). These synchronized retinal spikes have been postulated to arise via connectional mechanisms. One such mechanism is gap junction coupling, in which synchronous spikes would be a by-product of lateral signal shared by electrical coupling among ganglion cells (< 1 ms jitter). Synchronous spikes have also been postulated to arise from common-source inputs to retinal ganglion cells coming from electrical coupling with amacrine connections having overlapping receptive fields, and thus coding for stimulus location in the overlapping area (10-50 ms jitter). Finally, on a broad timescale (40–100 ms), common inputs from photoreceptors transmitted to the ganglion cell layer via chemical synapses are another source of activity synchronization (Brivanlou et al. 1998; DeVries 1999). Thus, the concerted-firing strategy is most effective when firing rates are low, so that coincidences due to chance are relatively infrequent. A recent report explores correlated firing among neighboring, directionally selective, ganglion cells as a function of stationary flashes versus moving spots and extended bars. The results showed that movement of a spot tends to increase the correlation in firing over that produced by flashes, and movement of an extended contour produces more correlated firing than small moving spots, emphasizing the importance of the stimulus feature (Amthor et al. 2005). On the other hand, Nirenberg and colleagues postulated that retinal synchronization, although it occurs, may be unimportant as an encoding mechanism, because more than 90% of the transmitted information about natural stimuli could be obtained from ganglion cells of the mouse retina while ignoring their correlated firing, indicating that ganglion cells act largely independently to encode information (Nirenberg et al. 2001).
However, these studies on synchrony or reliability of neuronal firing were designed to study the local processing of information inside the retina. In general, this research did not focus on the interactions with subsequent relay structures and their impact on encoding mechanisms dependent on coincidence detection or cross-correlation in neural activity. In summary, retinal ganglion cells respond in a precise, temporal fashion to some properties of the visual stimuli, modulating their firing rates with a speed of change as low as 1 ms. Nevertheless, it seems likely that synchronization among these cells can enhance the temporal integration at the next level of processing. For example, it is well known that 5–10% of the input to the lateral geniculate relay cells derives from the retina, which is the driving input; the rest of the input is modulatory and derives from local inhibitory inputs, descending inputs from layer 6 of the visual cortex, and ascending inputs from the brainstem. This input controls many features of retinogeniculate transmission (Sherman and Guillery 2002). Researchers have examined the role that spike timing of retinal afferents plays in driving thalamic and cortical responses, using multiple extracellular recordings. This arrangement allows for a detailed comparison of the lateral geniculate nucleus (LGN) response and its retinal input, and it makes the relay neuron of the LGN an important model system in which to study the regulation of sensory transmission from the periphery to the cortex (Victor 1999).
An interesting property of timed visual responses in the retina is the paired-spike enhancement. For a pair of retinal spikes from a single ganglion cell with a very short inter-spike interval (ISI), i.e. within less than 30 ms of each other, in vivo experiments have demonstrated that a second spike in the train is about 12 times more likely than the first to produce a LGN spike; at ISIs greater than 30 ms, second retinal spike are equal to the first spike in their probability of producing an LGN action potential (Usrey et al. 1998; Levine and Cleland 2001). Thus, it is possible that small groups of correlated ganglion cells sending convergent afferents to a single LGN neuron may mimic the paired-spike enhancement effect, employing temporal spike summation. Information encoded in the high firing rate of an individual retinal ganglion cell becomes distributed among several LGN neurons that fire synchronously. Then, synchrony according to anatomical divergence in the LGN is both strong and fast: up to 30% of the spikes from LGN cells that receive input from the same retinal ganglion cell can occur within less than 1 ms of each other, supporting the notion that LGN synchrony plays a major role in visual processing. In other words, there is a partial transformation of a single-cell rate code to a population temporal code.
The LGN is the main source of afferent input to the primary visual cortex, where single, simple cells in layer 4 receive convergent inputs from a very specific pool of at least 30 LGN cells. This convergence can be used by cortical neurons to identify precise temporal correlations between thalamic inputs, and therefore, it is a candidate mechanism to transmit information from one level to the next in the hierarchy (Reid 2001; Kara and Reid 2003). At the divergence side, a single, magnocellular thalamic cell can target as many as 400 cortical cells. Because of this, one is driven to suspect that the neural code for vision changes dramatically at this stage (Lestienne 2001). This interplay of anatomy and physiology acts not only to reinforce the pathway from the periphery to the cortex, but also to provide the cortex with more information about the visual environment (Dan et al. 1998; Rossi and Paradiso 1999). Simultaneous recordings in the LGN and the cortex have in fact shown that synchronous spikes from the LGN act synergistically in driving their cortical targets, and the effect decreases with ISIs up to 15 ms (Usrey et al. 2000). Such coincidence-detection mechanisms in the postsynaptic cortical cell may provide a means for reading out the population temporal code found in the LGN. This synergistic theory has recently been supported by means of intracellular measurements in an intact brain, pointing out that a group of just 30 synchronized inputs will drive the activity of one cortical neuron in layer four of V1 (Bruno and Sakmann 2006).
Theories of temporal coding through visual cortical networks are more diverse, given that visual areas are extensively interconnected by pathway convergence and divergence, as well as by lateral and feedback projections. Visual areas consist of a spatially distributed, temporally overlapped, and hierarchically organized network that processes information in parallel, which makes it difficult to crack their intrinsic dynamics (Knudsen et al. 1987; Felleman and Van Essen 1991; Schmolesky et al. 1998). The existence of reciprocal connections between cortical areas suggests that the most common informational transaction may be the recursive exchange of information between areas, rather than its unidirectional transfer from one area to another (Bressler 1996). However, the possibility of spatiotemporal spike coding on the basis of spike timing, synchronization, and mutual correlation of spikes from different neurons is currently being explored (Eckhorn 1994; Salinas and Sejnowski 2001).
Cortical neurons can temporally represent stimulus properties by means of two broad strategies: stimulus-driven temporal correlations (when coding; Fig. 2d top) and stimulus-triggering of endogenous temporal-response patterns (what is encoding; Fig. 2d bottom). Temporal coding of a signal is characterized by a one-to-one correspondence between the time of occurrence of a sensory event and the time of occurrence of the corresponding neural influx or phase-locked response (Merchant et al. 2004), whereas temporal encoding of a signal corresponds to situations in which information about static or dynamic signals is encoded in the temporal pattern of spikes (temporal encryption) without the spikes being tied to changes in the signal itself (Lestienne 2001). The former has been evaluated for the reliability of the average spike rate of a group of cortical neurons, representing a time-varying signal like the critical flicker frequency, assessing the limits of the temporal fidelity of cortical spike rate signals (Wells et al. 2001). On the other hand, encoding is well exemplified, given that activated neuronal groups possess the intrinsic property to oscillate; these oscillations constitute rhythmic modulations in neuronal excitability that affects both the likelihood of spike output and the sensitivity to synaptic input. Thus, rhythmic excitability peaks constitute rhythmically reoccurring temporal windows for communication. Only coherently oscillating (or phase-locked) neuronal groups can communicate effectively, because their communication windows for input and for output are open at the same times. (Buzsaki and Draguhn 2004; Fries 2005).
In the early stages of visual processing, objects and scenes are represented by neurons with small visual receptive fields. Each neuron provides information about local features of a scene, but to describe a scene, information must be integrated across the visual field and combined according to specific attributes (Shadlen and Movshon 1999). One theory has proposed that perceptual grouping and figure ground discriminations are represented dynamically by the formation of cell assemblies (Fig. 4), which are themselves defined by the fast correlations or possibly synchronization of distributed neuronal activities on a millisecond time scale (von der Malsburg 1981; Altmann et al. 1986). This spiking behavior usually coincides with a large-scale oscillatory background, but it does not depend of its presence (Eckhorn 1994; Roy et al. 2001). Many investigators believe that neuronal synchronization is critical for transmitting sensory information and have suggested that a major function of cortical neurons is to detect coincident events among their presynaptic inputs (Abeles 1991; Kreiter 2001). Neuronal synchronization is present in many brain regions during sensory stimulation, but its role in sensory processing is controversial (Gray 1999; Shadlen and Movshon 1999).
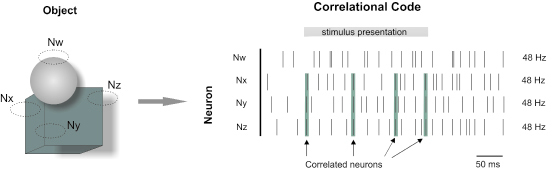
Figure 4. Object-background segmentation by time-dependent correlation of multiple neurons. One of two superposed objects was discriminated by three neurons (Nx, Ny and Nz) sensitive to vertical contours falling within their receptive fields. These three neurons shows coupled spiking activity binding the object properties, while their firing rates remain constant.
Cross-correlation studies performed in cat visual cortex have shown that neurons in different cortical areas of the same hemisphere or in corresponding areas of opposite hemispheres tend to synchronize their activities. Cross-correlation and auto-correlation functions from simultaneous recordings in areas 17 (V1) and 18 (V2) of anaesthetized cats responding to a stationary or moving stimulus of variable frequency, showed firing patterns phase-locked to the frequency of the ongoing stimuli at different recording sites and between them. Synchronizations were dominated by a cortical oscillating mechanism operating in the 30–60 Hz frequency range, activated preferentially with moving stimuli, and more frequent for cells in area 18 than in area 17. (Castelo-Branco et al. 1998; Rager and Singer 1998). Accordingly, paired recordings in V1 and V2 of paralyzed and anesthetized macaque monkeys in response to moving and flashed bars, have demonstrated that synchronization also occurs between the two areas, but near zero phase-lag correlations were rare (Nowak et al. 1999). In alert animals, millisecond synchronizations and gamma-band activity (20-70 Hz) in the striate cortex was strongly dependent on visual stimulation and is largely absent during spontaneous activity. In addition, the frequency of gamma-band activity also reflected stimulus properties, with drifting gratings evoking higher-frequency oscillations than stationary gratings (Livingstone 1996; Friedman-Hill et al. 2000; Maldonado et al. 2000). Another set of studies have stressed the importance of highly reproducible spike patterns and oscillations in extrastriate visual cortical areas of awake monkeys, where information about stimulus features is computed to bring out a particular percept. For example, this is the case for the medial temporal area (MT), a region that plays a major role in processing motion information and is at least five synaptic stages away from the sensory input (Salzman et al. 1990). When MT neurons were studied with time varying stimuli, 80% of cells were capable of responding with jitter from 2 ms to 10 ms, and about 62% of the cells showed an activity peak in the 20-60 Hz frequency band. These studies have confirmed that extrastriate neurons in alert primates can encode the fine temporal structure of visual stimuli (Bair et al. 1994; Bair and Koch 1996; Buracas et al. 1998). This approach has been used to assess the reproducibility of spike trains in response to a more naturalistic input, which provides strong evidence that visual stimuli can synchronize neurons on the time scale of several milliseconds.
Now we turn to the relationship between correlated firing and a specific function. Perhaps the strongest evidence that oscillations and synchrony in the gamma-band are involved in a specific cognitive process comes from V4 recordings on awake behaving monkeys, in which the visual input is kept fixed, while the monkey attention shifts to different parts of the visual scene. Neurons activated by the attended stimulus showed increased gamma-frequency (35-90 Hz) synchronization but reduced low-frequency (17 Hz) synchronization compared with neurons at nearby V4 sites activated by distracters (Fries et al. 2001). It should be noted that that serial and parallel mechanisms of response enhancement and neural synchrony work together to identify objects in a scene (Bichot et al. 2005). An interesting and representative case of synchronicity has been investigated on binocular rivalry; that is, when the images in the two eyes are incongruent and cannot be fused into a coherent percept, only signals from one of the two eyes are perceived, whereas those from the other eye are suppressed. The search for neuronal correlates in the cat primary visual cortex has shown an increase in the synchrony of cells when the signals conveyed passed from being suppressed to being perceived (Fries et al. 1997). Recently, it was reported that behavioral response times to a stimulus change can be predicted specifically by the degree of gamma-band synchronization among those neurons in monkey visual area V4 that are activated by the behaviorally relevant stimulus, reflecting an early neuronal correlate of efficient visuo-motor integration (Womelsdorf et al. 2006). The similarities in the properties of synchronous oscillations in the monkey and cat suggest that this form of neuronal activity is a general property of mammalian striate cortex. The above findings have been extended by the demonstration, based on gamma oscillation in humans, which only face perception induces a long-distance pattern of synchronization corresponding to the moment of perception itself and to the ensuing motor response. A period of strong de-synchronization marks the transition between the moment of perception and the motor response (Rodriguez et al. 1999).
The results reviewed so far provide correlative evidence for a role of response synchronization in neuronal processing, but they permit no stringent inferences as to whether the nervous system ascribes meaning to the precise temporal correlations among discharges. An attractive feature of this temporal coding strategy is that ensembles can be highly dynamic, and different stimuli will create broad coherent neuronal groupings that dissolve and settle into new configurations (Langheim et al. 2006). Thus, in the visual system even entirely novel stimuli could be represented by the coherent activity of a particular ensemble. Finally, as we mention early, all presynaptic action potentials terminate at postsynaptic neurons, where they initiate postsynaptic currents that are integrated collectively to trigger or inhibit new spikes. But, who reads out the information? Or where are the representations implemented? The first stage of a postsynaptic cell is the dendritic arbor; on average each dendrite of a postsynaptic cell receives about 6000 presynaptic inputs. Each synapse selects a unique mélange of features of the presynaptic spikes and transmits only a specific subset of the information contained in the entire train. Different aspects of the same train are read out by different target cells; thus, although the spikes on one axon are identical events, their effects on a postsynaptic cell vary from spike to spike given the spatio-temporal dynamics of the dendritic field (Gerstner et al. 1997).
No study, to our knowledge, has directly addressed the potential of a dendritic field for representation. Nevertheless, a novel hypothesis (Markram 2006) proposed that neural microcircuits construct multidimensional electrical objects on 3D co-ordinates of all dendritic segments from all neurons within a volume of brain tissue. In this theory, action potentials are used to produce voltage responses in dendrites in order to construct and maintain 3D electrical objects that span continuously across all dendritic segments in the neural volume. Synaptic properties are tuned to allow each neuron to contribute a unique “electrical trait”, and the local recurrent circuitry is used to merge and integrate these “electrical traits” into meaningful “electrical objects” that represent the stimulus. Also suggested was that 3D dendritic object formation is a generic capability of all neural microcircuits and that specialization of brain regions allows merging and integration of elementary electrical objects formed in local microcircuits into more complex objects and eventually into complete scenes of the world. So, the transference of information from one node to the next compels minimization of the number of spikes used as we learn to transfer just the required information. We are thus led to a view of neural coding that is quite distinct from the classical picture of information processing based solely on action potential patterns.
CONCLUDING REMARKS
Much information about the world is embedded in time, and recent advances in neuroscience have revealed the major significance of temporal coding in the brain and its indispensable role in neural information processing. The distribution patterns of these temporal codes across the cortical surface and subcortical structures give an indication of how conserved is this representational mechanism, which may have evolved to optimize the properties of the network’s circuitry. The picture emerging from this review allows for the following conclusions.
First, temporal codes can be viewed in two different contexts: temporal neural discharges simply follow the temporal variations of the stimulus, and spike timing thus provides information about the occurrence of a change in the stimulus with certain accuracy (phase-locked coding). A different context is one in which temporal firing patterns do not result directly from the time-varying features of the stimulus. Rather, such patterns are a product of brain circuit dynamics (intrinsic encoding). This dichotomy may be a useful heuristic to identify critical variables driving each of the two functional states. Second, response variability is a property of synaptic connections, not of neurons themselves (Movshon 2000). However, sparse activations are also capable of representing simultaneously the enormous complexity and variability of the natural environment, in properly configured neural networks. For example, individual neurons in area MT of alert monkeys can discriminate better between stimuli with rich temporal structure than constant-motion stimuli that differ only in direction (Buracas et al. 1998). Third, using a simple rate coding and independent pooled neurons it has been shown that the time needed for several spikes to accumulate in order to estimate the firing rate is usually longer than what is needed for most perceptual or behavioral processes. From this, we stress the necessity of coupling simultaneous extracellular multiunit recordings (output) with optical imaging techniques (input) in order to elucidate the true nervous system dynamics. Voltage-sensitive dyes (VSDs) insert into the plasma membrane and change their fluorescence intensity dependent on the potential across the lipid bilayer. Some VSDs has proven useful, allowing the spatiotemporal analysis of electrical signaling in dendrites (input). Extracellular multiunit recordings capture the suprathreshold activity of neurons, typically spikes at soma or along the axon (output). Fourth, correlated or near synchronous neuronal activity of the same assembly with a precision in the microsecond to millisecond range has been described as an independent channel of information flow. Experimental studies mentioned above indicate that large variations in correlations can be observed in the absence of simultaneous variations in mean firing rates. Rate-independent modulations in synchrony have been linked to changes in expectation, attention, response latency, and rivalry, all of which process and adjust the flow of information (Stuart et al. 2005). These internal processes are likely to be modulated by top-down feedback pathways that strongly shape the intrinsic dynamics of thalamocortical networks and constantly create predictions about forthcoming sensory events (Engel et al. 2001).
We can conclude that several mechanisms are available to cortical neurons that allow them to generate and to respond to concerted activity as part of their everyday dynamics, highlighting the fact that information processing in the neuronal circuitry depends to a large extent on how signals are channeled through the brain, and how the relevant circuitry can be quickly adapted to the current signal processing for the semantics of representation. It seems very likely that all play a part, but in which circumstances and which combinations remains to be determined.
ACKNOWLEDGMENTS
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) doctoral fellowship. We thank Luis Prado and Raul Paulin for their technical assistance, and Dorothy Pless and Amaya Miquelajauregui for their comments to earlier versions of the manuscript.
REFERENCES
Abeles M (1991) Corticonics: neural circuits of the cerebral cortex. Cambridge University Press
Abeles M, Bergman H, Gat I, Meilijson I, Seidemann E, Tishby N, Vaadia E (1995) Cortical activity flips among quasi-stationary states. Proc Natl Acad Sci USA 92: 8616-8620
Adrian ED (1928) The basis of sensation: the action of the sense organs. Christopher, London
Aertsen A, Braitenberg V (1996) Brain theory: biological basis and computational principles. Elsevier, Amsterdam
Altmann L, Eckhorn R, Singer W (1986) Temporal integration in the visual system: influence of temporal dispersion on figure-ground discrimination. Vision Res 26: 1949-1957
Amthor FR, Tootle JS, Grzywacz NM (2005) Stimulus-dependent correlated firing in directionally selective retinal ganglion cells. Vis Neurosci 22: 769-787
Arieli A, Sterkin A, Grinvald A, Aertsen A (1996) Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273:1868-1871
Bair W, Koch C (1996) Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput 8: 1185-1202
Bair W, Koch C, Newsome W, Britten K (1994) Power spectrum analysis of bursting cells in area MT in the behaving monkey. J Neurosci 14: 2870-2892
Berry MJ, Warland DK, Meister M (1997) The structure and precision of retinal spike trains. Proc Natl Acad Sci USA 94: 5411-5416
Bichot NP, Rossi AF, Desimone R (2005) Parallel and serial neural mechanisms for visual search in macaque area V4. Science 308: 529-534
Bressler SL (1996) Interareal synchronization in the visual cortex. Behav Brain Res 76: 37-49
Brivanlou IH, Warland DK, Meister M (1998) Mechanisms of concerted firing among retinal ganglion cells. Neuron 20: 527-539
Bruno RM, Sakmann B (2006) Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312: 1622-1627
Buracas GT, Zador AM, DeWeese MR, Albright TD (1998) Efficient discrimination of temporal patterns by motion-sensitive neurons in primate visual cortex. Neuron 20: 959-969
Buzsaki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304: 1926-1929
Castelo-Branco M, Neuenschwander S, Singer W (1998) Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J Neurosci 18: 6395-6410
Christodoulou C, Bugmann G (2001) Coefficient of variation (CV) vs mean inter-spike-interval (ISI) curves: what do they tell us about the brain? Neurocomputing 38-40: 1141-1149
Cohen MR, Newsome WT (2004) What electrical microstimulation has revealed about the neural basis of cognition. Curr Opin Neurobiol 14: 169-177
Dan Y, Alonso JM, Usrey WM, Reid RC (1998) Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci 1: 501-507
Dayan P, Abbott LF (2001) Theoretical neuroscience: computational and mathematical modeling of neural systems. MIT Press, Cambridge, MA
deCharms RC, Zador A (2000) Neural representation and the cortical code. Annu Rev Neurosci 23: 613-647
DeVries SH (1999) Correlated firing in rabbit retinal ganglion cells. J Neurophysiol 81: 908-920
Di Lorenzo PM, Hallock RM, Kennedy DP (2003) Temporal coding of sensation: mimicking taste quality with electrical stimulation of the brain. Behav Neurosci 117: 1423-1433
Eagleman DM, Churchland PS (in press) Ten Unsolved Questions of Neuroscience. The MIT Press, Cambridge
Eckhorn R (1994) Oscillatory and non-oscillatory synchronizations in the visual cortex and their possible roles in associations of visual features. Prog Brain Res 102: 405-426
Engel AK, Fries P, Singer W (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2: 704-716
Erickson RP (2001) The evolution and implications of population and modular neural coding ideas. Prog Brain Res 130: 9-29
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1-47
Friedman-Hill S, Maldonado PE, Gray CM (2000) Dynamics of striate cortical activity in the alert macaque: I. Incidence and stimulus-dependence of gamma-band neuronal oscillations. Cereb Cortex 10: 1105-1116
Fries P (2005) A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9: 474-480
Fries P, Reynolds JH, Rorie AE, Desimone R (2001) Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560-1563
Fries P, Roelfsema PR, Engel AK, Konig P, Singer W (1997) Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proc Natl Acad Sci USA 94: 12699-12704
Friston KJ (1997) Another neural code? Neuroimage 5: 213-220
Furukawa S, Middlebrooks JC (2002) Cortical representation of auditory space: information-bearing features of spike patterns. J Neurophysiol 87: 1749-1762
Galambos R, Davis H (1948) Action potential from single auditory nerve fibers. Science 108: 513
Georgopoulos AP, Schwartz AB, Kettner RE (1986) Neuronal population coding of movement direction. Science 233: 1416-1419
Gerstner W, Kreiter AK, Markram H, Herz AV (1997) Neural codes: firing rates and beyond. Proc Natl Acad Sci USA 94: 12740-12741
Gray CM (1999) The temporal correlation hypothesis of visual feature integration: still alive and well. Neuron 24: 31-47, 111-125
Guyonneau R, Vanrullen R, Thorpe SJ (2004) Temporal codes and sparse representations: a key to understanding rapid processing in the visual system. J Physiol Paris 98: 487-497
Halpern BP (2000) Sensory coding, decoding, and representations. Unnecessary and troublesome constructs? Physiol Behav 69: 115-118
Hernandez A, Zainos A, Romo R (2002) Temporal evolution of a decision-making process in medial premotor cortex. Neuron 33: 959-972
Hess RF, Hayes A, Field DJ (2003) Contour integration and cortical processing. J Physiol Paris 97: 105-119
Holt GR, Softky WR, Koch C, Douglas RJ (1996) Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J Neurophysiol 75: 1806-1814
Hubel DH, Wiesel TN (1959) Receptive fields of single neurones in the cat's striate cortex. J Physiol 148: 574-591
Johansson RS, Birznieks I (2004) First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci 7: 170-177
Kara P, Reid RC (2003) Efficacy of retinal spikes in driving cortical responses. J Neurosci 23: 8547-8557
Kara P, Reinagel P, Reid RC (2000) Low response variability in simultaneously recorded retinal, thalamic, and cortical neurons. Neuron 27: 635-646
Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A (2003) Spontaneously emerging cortical representations of visual attributes. Nature 425: 954-956
Knight BW (1972) Dynamics of encoding in a population of neurons. J Gen Physiol 59: 734-766
Knoblauch A, Palm G (2005) What is signal and what is noise in the brain? Biosystems 79: 83-90
Knudsen EI, du Lac S, Esterly SD (1987) Computational maps in the brain. Annu Rev Neurosci 10: 41-65
Kreiter AK (2001) Functional implications of temporal structure in primate cortical information processing. Zoology (Jena) 104: 241-255
Langheim FJ, Leuthold AC, Georgopoulos AP (2006) Synchronous dynamic brain networks revealed by magnetoencephalography. Proc Natl Acad Sci USA 103: 455-459
Lee D, Port NL, Kruse W, Georgopoulos AP (1998) Variability and correlated noise in the discharge of neurons in motor and parietal areas of the primate cortex. J Neurosci 18: 1161-1170
Lennie P (2003) The cost of cortical computation. Curr Biol 13: 493-497
Lestienne R (2001) Spike timing, synchronization and information processing on the sensory side of the central nervous system. Prog Neurobiol 65: 545-591
Levine MW, Castaldo K, Kasapoglu MB (2002) Firing coincidences between neighboring retinal ganglion cells: inside information or epiphenomenon? Biosystems 67: 139-146
Levine MW, Cleland BG (2001) An analysis of the effect of retinal ganglion cell impulses upon the firing probability of neurons in the dorsal lateral geniculate nucleus of the cat. Brain Res 902: 244-254
Livingstone MS (1996) Oscillatory firing and interneuronal correlations in squirrel monkey striate cortex. J Neurophysiol 75: 2467-2485
Luna R, Hernandez A, Brody CD, Romo R (2005) Neural codes for perceptual discrimination in primary somatosensory cortex. Nat Neurosci 8: 1210-1219
Mainen ZF, Sejnowski TJ (1995) Reliability of spike timing in neocortical neurons. Science 268: 1503-1506
Maldonado PE, Friedman-Hill S, Gray CM (2000) Dynamics of striate cortical activity in the alert macaque: II. fast time scale synchronization. Cereb Cortex 10: 1117-1131
Markram H (2006) Dendritic object theory: a theory of the neural code where 3D electrical objects are formed across dendrites by neural microcircuits. In: Swiss Soc. Neurosci. Abstr, Basel
Mauk MD, Buonomano DV (2004) The neural basis of temporal processing. Annu Rev Neurosci 27: 307-340
Meister M, Berry MJ, 2nd (1999) The neural code of the retina. Neuron 22: 435-450
Merchant H, Battaglia-Mayer A, Georgopoulos AP (2004) Neural responses in motor cortex and area 7a to real and apparent motion. Exp Brain Res 154: 291-307
Moore DR, Schnupp JW, King AJ (2001) Coding the temporal structure of sounds in auditory cortex. Nat Neurosci 4: 1055-1056
Mountcastle VB (1980) Neural mechanisms in somesthesis. In: Mountcastle VB (ed) Medical Physiology, vol 1. Mosby, London, UK pp 348-390
Movshon JA (2000) Reliability of neuronal responses. Neuron 27: 412-414
Nirenberg S, Carcieri SM, Jacobs AL, Latham PE (2001) Retinal ganglion cells act largely as independent encoders. Nature 411: 698-701
Nowak LG, Munk MH, James AC, Girard P, Bullier J (1999) Cross-correlation study of the temporal interactions between areas V1 and V2 of the macaque monkey. J Neurophysiol 81: 1057-1074
Paillard J (1983) The functional labeling of neural codes. Exp Brain Res 7: 1-19
Panzeri S, Petersen RS, Schultz SR, Lebedev M, Diamond ME (2001) The role of spike timing in the coding of stimulus location in rat somatosensory cortex. Neuron 29: 769-777
Panzeri S, Pola G, Petersen RS (2003) Coding of sensory signals by neuronal populations: the role of correlated activity. Neuroscientist 9: 175-180
Parker AJ, Newsome WT (1998) Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci 21: 227-277
Rager G, Singer W (1998) The response of cat visual cortex to flicker stimuli of variable frequency. Eur J Neurosci 10: 1856-1877
Reich DS, Mechler F, Purpura KP, Victor JD (2000) Interspike intervals, receptive fields, and information encoding in primary visual cortex. J Neurosci 20: 1964-1974
Reid RC (2001) Divergence and reconvergence: multielectrode analysis of feedforward connections in the visual system. Prog Brain Res 130: 141-154
Rieke F, Warland D, van Stevenink R, and Bialek W (1997) Spikes: exploring the neural code. The MIT Press, Massachusetts
Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ (1999) Perception's shadow: long-distance synchronization of human brain activity. Nature 397: 430-433
Rolls ET (2000) Functions of the primate temporal lobe cortical visual areas in invariant visual object and face recognition. Neuron 27: 205-218
Romo R, Hernandez A, Zainos A, Salinas E (1998) Somatosensory discrimination based on cortical microstimulation. Nature 392: 387-390
Ronacher B, Franz A, Wohlgemuth S, Hennig RM (2004) Variability of spike trains and the processing of temporal patterns of acoustic signals-problems, constraints, and solutions. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190: 257-277
Rossi AF, Paradiso MA (1999) Neural correlates of perceived brightness in the retina, lateral geniculate nucleus, and striate cortex. J Neurosci 19: 6145-6156
Roy SA, Dear SP, Alloway KD (2001) Long-range cortical synchronization without concomitant oscillations in the somatosensory system of anesthetized cats. J Neurosci 21: 1795-1808
Sakurai Y (1996) Population coding by cell assemblies--what it really is in the brain. Neurosci Res 26: 1-16
Salinas E, Hernandez A, Zainos A, Romo R (2000) Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci 20: 5503-5515
Salinas E, Sejnowski TJ (2001) Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2: 539-550
Salzman CD, Britten KH, Newsome WT (1990) Cortical microstimulation influences perceptual judgements of motion direction. Nature 346: 174-177
Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG (1998) Signal timing across the macaque visual system. J Neurophysiol 79: 3272-3278
Shadlen MN, Movshon JA (1999) Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron 24: 67-77, 111-125
Shadlen MN, Newsome WT (1994) Noise, neural codes and cortical organization. Curr Opin Neurobiol 4: 569-579
Shadlen MN, Newsome WT (1998) The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 18: 3870-3896
Sherman SM, Guillery RW (2002) The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357: 1695-1708
Softky WR, Koch C (1993) The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci 13: 334-350
Stein RB, Gossen ER, Jones KE (2005) Neuronal variability: noise or part of the signal? Nat Rev Neurosci 6: 389-397
Steinmetz PN, Manwani A, Koch C (2001) Variability and coding efficiency of noisy neural spike encoders. Biosystems 62: 87-97
Stevens CF, Zador AM (1998) Input synchrony and the irregular firing of cortical neurons. Nat Neurosci 1: 210-217
Stuart L, Walter M, Borisyuk R (2005) The correlation grid: analysis of synchronous spiking in multi-dimensional spike train data and identification of feasible connection architectures. Biosystems 79: 223-233
Thorpe S, Delorme A, Van Rullen R (2001) Spike-based strategies for rapid processing. Neural Netw 14: 715-725
Thorpe S, Fize D, Marlot C (1996) Speed of processing in the human visual system. Nature 381: 520-522
Tolhurst DJ, Movshon JA, Dean AF (1983) The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res 23: 775-785
Usrey WM, Alonso JM, Reid RC (2000) Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. J Neurosci 20: 5461-5467
Usrey WM, Reppas JB, Reid RC (1998) Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature 395: 384-387
Victor JD (1999) Temporal aspects of neural coding in the retina and lateral geniculate. Network 10: R1-66
Vogel A, Hennig RM, Ronacher B (2005) Increase of neuronal response variability at higher processing levels as revealed by simultaneous recordings. J Neurophysiol 93: 3548-3559
von der Malsburg C (1981) The correlation theory of brain function. In: Max-Planck-Institute for Biophysical Chemistry, Internal Report 81-2, Goettingen, pp 1-26 [Reprinted in: Domany E, van Hemmen JL, Schulten K (eds) (1994) Models of Neural Networks II. Springer, Berlin, Germany]
Wells EF, Bernstein GM, Scott BW, Bennett PJ, Mendelson JR (2001) Critical flicker frequency responses in visual cortex. Exp Brain Res 139: 106-110
Werner G, Mountcastle VB (1963) The variability of central neural activity in a sensory system, and its implications for the central reflection of sensory events. J Neurophysiol 26: 958-977
Womelsdorf T, Fries P, Mitra PP, Desimone R (2006) Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439: 733-736
Wright BD, Sen K, Bialek W, Doupe AJ (2002) Spike timing and the coding of naturalistic sounds in a central area of songbirds. In: Dietterich G, Becker S, Ghahramani Z (eds) Advances in Neural Information Processing Systems, vol 14. MIT Press, Cambridge, MA, pp 309-316